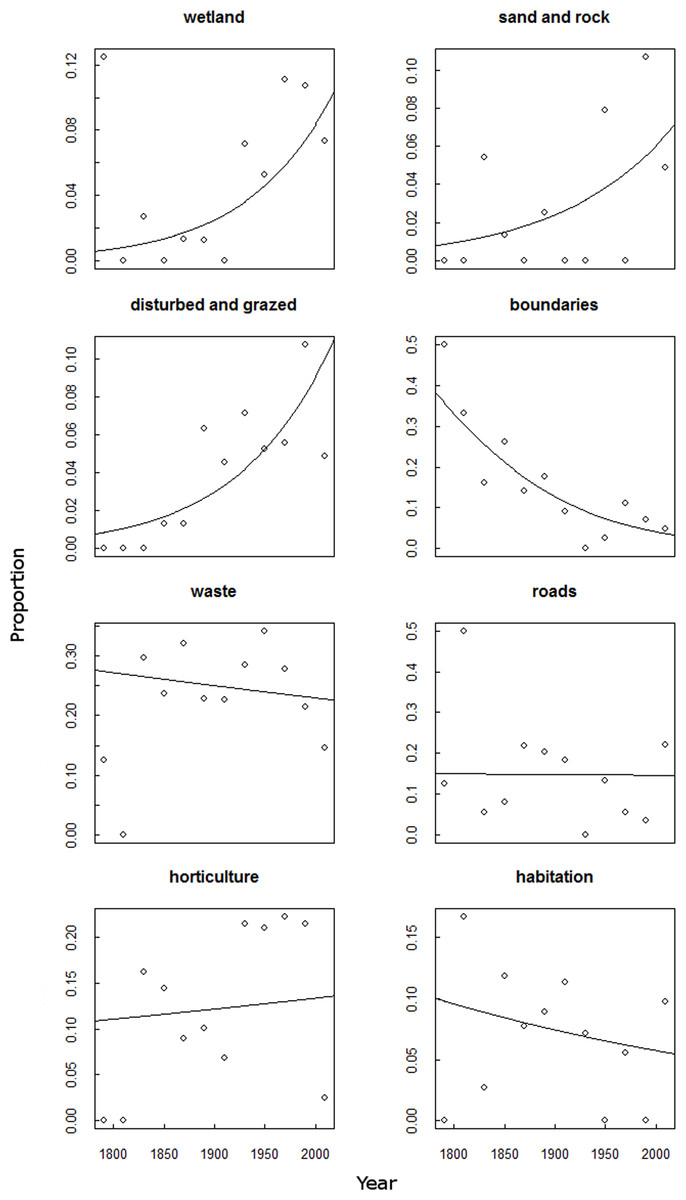
Many
people remark on the changes that are occurring in the countryside, the
disappearance of some species and the spread of others. Yet these anecdotes
cannot substitute for hard facts. There are also many suggested causes for all
these changes; a warmer climate, different agricultural practises,
eutrophication, alien species etc. Botanical observations tend to be biased. For
example, people often note the exceptional species but ignore the common ones.
So it is difficult to draw conclusions about plant abundance from casual
observations. What was needed was a dedicated survey with a clear repeatable
methodology.
Common
plant species are the mainstay of habitats, they create our woodlands,
hedgerows and
meadows; they provide the food for herbivores and pollinators and
they create homes for birds and mammals. Changes in the abundance of rare
species have little impact on other species, but change in the abundance of
common species can have cascading effects on whole ecosystems of which we are a
part.
 |
| The distribution of heather in Durham and South Northumberland predicted from the common plant survey data. |
For
these reasons volunteer botanists in the north-east of England conducted a four
year survey to benchmark the abundance of common plants. Led by the Botanical
Societies vice county recorders, John Durkin, John Richards and Quentin Groom
they surveying the plants in a randomly selected sample of 1km2 grid
squares in the vice counties of Durham and South Northumberland. They have created a solid foundation that can
be used to qualify the abundance of common species and be compare against previous
and future studies. The project was conducted over four years and required
volunteers to go to all sorts of places. Some people surveyed post-industrial
brown-field sites, while other walked for miles across bleak moorland to reach sites
high in the hills. Although, these moors are arguable more wild and natural,
the industrial wastelands are far more biodiverse.
The
results of this survey have just been openly published, contributing an
additional 35,000 observations to the 200,000 observations collected by local
recorders since the turn of the millennium (http://bdj.pensoft.net/articles.php?id=7318).
Botanical
surveying continues in the region despite the end of this project. Volunteers
continue to monitor rare plants in the region (https://dx.doi.org/10.6084/m9.figshare.1480492; http://www.bsbi.org.uk/County_Durham_Rare_Plants_Register_2013.pdf) and are currently
working towards the next atlas of Britain and Ireland coordinated by the
Botanical Society of Britain and Ireland (http://bsbi.org.uk/).
Good
biological conservation in the 21st century will be as much to do
with sensitive adaption to change as it is about preserving what we have. Human
memory is short and fickle and it is only with benchmark surveys, such as this,
that we can hope to understand and manage that change.